In an industry built on caution, moving fast isn’t just difficult—it’s radical. Biopharma is known for its complexity, long development cycles, and uncompromising regulatory standards. It’s not exactly fertile ground for agility, let alone transformation at scale. But at Sanofi, the rules are being rewritten.
Headquartered in Paris, Sanofi is a global healthcare company on a mission to chase the miracles of science to improve people’s lives. Operating in over 60 countries with more than 80,000 employees worldwide—and with approximately $46 billion (USD) in revenue in 2024—the company’s work touches more than 500 million lives annually. From breakthrough biologics to world-leading vaccines, the company develops treatments for chronic conditions and rare diseases with no existing therapies, and protects communities through immunizations against influenza, RSV, and meningitis.
Rooted in deep scientific expertise, Sanofi has evolved from a traditional pharmaceutical manufacturer into a research-driven biopharma leader with a growing presence in immunology, oncology, and gene therapy. Furthermore, the company has sharpened its focus—divesting from non-core businesses and investing in AI and R&D, and committing to lead in areas of high unmet medical need.
Importantly, the company isn’t targeting incremental improvements, it’s fundamentally changing how manufacturing works in one of the world’s most regulated, risk-sensitive sectors. Think digital twins that simulate entire production lines before a single machine is installed. AI-powered systems that boost yield on every batch. Modular “smart factories” that pivot between products in days instead of months. These aren’t future-state concepts—they’re already in play.

Facing a pipeline of more than 40 potential product launches by 2030, executive vice president of manufacturing and supply, Brendan O’Callaghan, is on a mission to streamline timelines, increase throughput, and digitize global operations—while ensuring Sanofi still delivers flawlessly, every time.
Recently, Mr. O’Callaghan sat down with IQ to share how Sanofi is marrying AI and automation with the precision of Formula 1 and the discipline of pharma.
The result? A bold new operating model that’s faster, smarter, and more resilient—built not just to survive disruption, but to thrive in it. For leaders navigating complexity, this isn’t just a story of transformation, it’s a glimpse into the future of enterprise agility.
IQ: Sanofi could potentially launch over 40 new products by 2030. That’s an extraordinary pace for a company in such a tightly regulated sector. Why now—and what does that acceleration demand of manufacturing?
Mr. O’Callaghan: It’s true—this level of ambition is unprecedented in our space. We’re looking at over 40 potential product launches in the next five years. That’s not just a pipeline goal—it’s our operational reality. And the role of manufacturing and supply is to take those scientific breakthroughs and make them real in order to get them into the hands of the patients who need them.
To make that happen, every second counts. We’re no longer talking about ramping up one or two launches per year. We’re talking about executing four, maybe even six, in a single year—each with its own complexity, its own regulatory path, and its own market demand. So we have to fundamentally change how we operate. We have to be faster, more agile, and infinitely more connected across our supply chain.
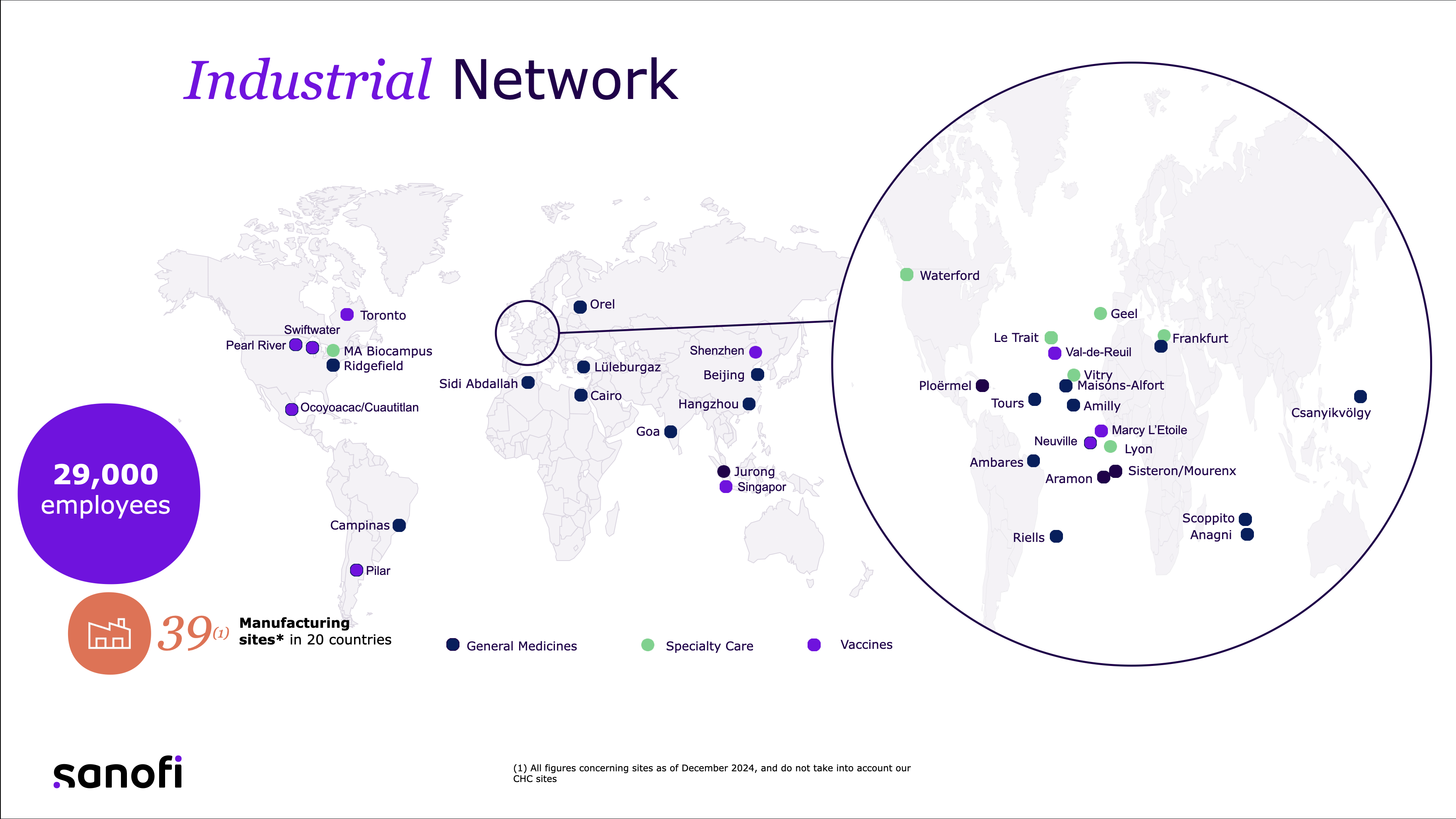
And we’re not doing this from a standing start. Today, our network includes thousands of employees across 39 sites worldwide, producing over 2 billion doses annually. But to hit the future we envision, we’re transforming that entire network—digitally, operationally, and culturally. Because speed without safety or quality isn’t an option in healthcare. Everything we do is built on the foundation of reliability, safety, and compliance.
IQ: Sanofi recently opened next-generation “Modulus” facilities in France and Singapore. How do these sites represent a shift in how you think about agility in manufacturing?
Mr. O’Callaghan: The Modulus sites are, in many ways, our blueprint for the future. They’re modular, flexible, and digitally enabled end-to-end. These aren’t just new buildings—they’re a complete rethinking of what biopharma production can be.
What makes them different is the level of agility. Traditional sites are built to manufacture one product—or one class of products—at scale. Switching between platforms or product types takes months, sometimes years. But at Modulus, we can switch between different vaccine or biologic platforms in a matter of days or weeks. The sites are designed as networks of mini-factories, 34 in one location, all of which can be configured and reconfigured like Lego building blocks, depending on demand and product mix.
So now, instead of building a new plant for every new therapeutic, we can scale within the same footprint. That’s how you respond to uncertainty. That’s how you meet demand in real time without compromising on quality or compliance.
IQ: You mentioned digital enablement—what role does AI play in driving that level of agility and scale?
Mr. O’Callaghan: AI is absolutely at the heart of it. In May, we launched what we call our Digital Manufacturing & Supply Accelerator in Lyon. The whole idea is to bring together experts in AI, data science, and process engineering to identify high-value use cases—and then industrialize them at speed.
One example is our use of digital twins. This allows us to simulate an entire production process before the line is even physically built. You can put on a VR headset, walk through the plant, see the flow of materials, and test for bottlenecks or risks. We’ve found this can shave two to three months off the deployment timeline for new lines.
Another innovation is SimplY—our proprietary, AI-powered system that uses telemetry from sensors across our bioreactors and production lines. It tracks thousands of data points in real time, which allows us to optimize yield and performance batch by batch. We’ve seen gains of 5% to 10% in production yield. That’s not theoretical—those are extra doses that reach patients faster. That’s how AI becomes not just a tool, but a driver of patient impact.
IQ: With AI evolving so rapidly, how do you balance the pressure to move fast with the rigorous safety and compliance expectations in biopharma? Mr. O’Callaghan: That’s a critical question for us; safety and quality aren’t just priorities—they’re non-negotiables. The medicines and vaccines we produce affect lives, so we build everything around that reality. In a sense, regulation is not a barrier—it’s a framework that helps guide innovation in the right direction.
AI can help us move faster, yes—but only if we apply it responsibly. That’s why we’ve embedded a human-in-the-loop principle into every AI deployment. AI helps detect deviations, analyze quality data, or recommend corrective actions—but the final call is always human. We also ensure our models are explainable and auditable. There’s no black box.
Our Responsible AI policy includes fairness, accountability, robustness, and sustainability. And this isn’t just internal policy—it’s operationalized. We won’t put any AI into production unless it passes ethical, regulatory, and cybersecurity checks. So, while the pressure to move fast is real, the risk of moving blindly is greater. We aim to move fast—but with eyes wide open.
IQ: I know you believe that it’s not just about tech—it’s also about people. What challenges have you faced in bringing manufacturing veterans and digital talent together?
Mr. O’Callaghan: That’s one of the biggest cultural shifts we’re navigating. In our plants, you have individuals with decades of experience in pharmaceutical operations—people who have literally written the playbook on how to run high-quality, validated manufacturing environments. And now we’re asking them to work alongside digital natives who come from tech, startups, or adjacent industries. These are people who move fast, iterate quickly, and think in terms of code and data.
The key has been mutual respect—and co-creation. We don’t parachute in digital solutions and expect them to work. We embed digital teams directly into the manufacturing context. We organize around what we call pods—small, interdisciplinary teams that include process engineers, operators, data scientists, and software developers. Everyone is accountable. Everyone learns from each other.

Of course, there are always growing pains. But there’s also a growing sense of shared purpose. When people realize that their ideas—whether from the shop floor or the code base—can materially impact how fast we get medicines to patients, something powerful happens. The silos start to dissolve.
IQ: As a follow-up, as you scale digital and AI initiatives, how are you ensuring that transformation reaches the frontline—across geographies, roles, and experience levels?
Mr. O’Callaghan: That’s where real transformation either lives or dies—in the hands of the people doing the work. It’s one thing to pilot a digital solution in a lab. It’s another to scale it across an organization operating in multiple countries, each with their own regulatory and cultural realities.
What we’ve learned is that you can’t “roll out” a mindset. You have to embed it. So we’ve structured our efforts to be deliberately inclusive and collaborative. Our pods allows for co-creation rather than top-down implementation.
We’ve also borrowed the two-pizza team model from the tech world—small enough to stay agile, but diverse enough to represent multiple views. And we’ve invested in digital fluency training across all levels. Because if people don’t understand the ‘why’ behind a tool, they won’t trust it or use it to its full potential.
The other piece is diversity. Our Sanofi digital accelerators include over 20 nationalities. That mix of perspectives helps us ensure that the tools we’re building work not just technically, but culturally—because the goal is not just adoption, it’s transformation.
IQ: Some of the gains you’ve mentioned are impressive—cutting changeover times by 40%, improving forecast accuracy by three percentage points. What metrics do you personally watch most closely?
Mr. O’Callaghan: Yield and cycle time are two that I watch closely, because they go directly to our ability to serve patients. If you can produce more doses per batch, that means more patients get treated. If you can shave months off tech transfer or product launch timelines, that means a parent waiting for a pediatric vaccine doesn’t have to wait quite as long.
We also track OEE—overall equipment effectiveness—and we’ve used AI and sensors to boost it across several of our sites. In some places, we’ve seen 20% to 25% improvements. And these aren’t marginal gains—they translate into significant savings and supply assurance.
But just as important are our quality and compliance metrics. In a regulated industry like ours, the cost of a mistake is too high. So we always maintain a human focus. AI can flag deviations, analyze trends, and even suggest corrective actions—but decisions are always reviewed and owned by our teams.
IQ: Looking ahead, what does “factory of the future” mean for Sanofi?
Mr. O’Callaghan: The vision is what we call a “lights-out factory.” That means a fully automated, self-optimizing manufacturing environment—where systems don’t just execute instructions, but learn, adapt, and improve on their own. But it’s not about replacing people. It’s about augmenting human capability.
We want our operators to spend less time on repetitive tasks and more time solving complex problems.
With connected systems and predictive analytics, a technician in Lyon can troubleshoot a yield issue before it becomes a problem.
A scientist in the lab can transfer their experiment to manufacturing virtually using a digital twin. That’s the power of what we call one-click tech transfer. Today, the tech transfer process—moving a process from R&D into manufacturing—can take months. We want to reduce that to weeks or even days. And we’re not starting from scratch—our Modulus sites are already showing us what’s possible. But the real opportunity is in scaling that model across the network and adapting it to different therapeutic modalities.
And when I say “lights out,” I don’t just mean automation. I mean reliability. I mean quality you can trust without needing manual intervention. I mean systems that continuously learn from themselves. That’s where we’re headed—and we’re building the capabilities now to make that real.
IQ: Lastly, in times of uncertainty and disruption, what anchors you? What gives you clarity?
Mr. O’Callaghan: For me, it always comes back to purpose. When you sit with patients—or with parents of sick children—you’re reminded very quickly why this work matters. We had a parent once look around the room during a leadership meeting and say, “You’re the smartest people at this company. Can’t you find something to help my son?”
That’s a sobering moment. It’s also deeply motivating. It forces you to ask: Are we doing everything we can? Are we moving fast enough?
So yes, the work is complex. And yes, we’re dealing with uncertainty—technological, regulatory, geopolitical. But if we keep the patient at the center, we usually find the right answer. That’s what gives me clarity. That’s what drives me every day.



